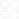
28120151ResearchofEnvironmentalSciencesVol.28No.1Jan.2015.UVFeⅢ-ⅡJ.201528182-87.SUNJieZENGPeiZHANGHan.DecolorizationofOrangeⅡinFeⅢ-fumaratesystemunderultravioletradiationJ.ResearchofEnvironmentalSciences201528182-87.2014-03-102014-08-142008CDB4002010518245671976-sunjie2076@hotmail.com.UVFeⅢ-Ⅱ430074FeⅢ-FeⅢ-Ⅱc〔FeⅢ〕、ρⅡcpHⅡ.pH3~7FeⅢ-ⅡpH.c〔FeⅢ〕25μmolL、ρⅡ25mgL、c250μmolL、pH3、50minⅡ100%TOC60%.Ⅱc〔FeⅢ〕cⅡ.Ⅱ.·OHⅡ.FeⅢ-Ⅱ·OHX7031001-6929201501-0082-06ADOI10.13198j.issn.1001-6929.2015.01.11DecolorizationofOrangeⅡinFeⅢ-FumarateSystemUnderUltravioletRadiationSUNJieZENGPeiZHANGHanInstituteofEnvironmentEngineeringandScienceSouth-CentralUniversityforNationalitiesWuhan430074ChinaAbstractPhoto-oxidationofthedyeOrangeⅡwasstudiedintheFeⅢ-fumaratesystemunderUVirradiation.TheeffectsofpHvalueandinitialconcentrationsofFeⅢfumarateandOrangeⅡwerealsoinvestigated.TheresultsshowedthatlowersolutionpHintherangeof3.0-7.0favorsthephotooxidationintheFeⅢ-fumaratesystemandtheremovalrateofOrangeⅡincreasedwithincreasinginitialconcentrationofFeⅢ.After50minutesthedecolorizationefficiencyofOrangeⅡandTOCsolutionsreached100%and60%respectivelywithpHwas3cFeⅢwas25μmolLcfumaratewas250μmolLandρOrangeⅡwas25mgL.TheeffectofinitialconcentrationsoffumaratewasnotobviousonthedecolorizationrateofOrangeⅡ.Underlaboratoryconditionsthephoto-oxidationofOrangeⅡfollowedthepseudofirstorderreaction.FluorescencemethodwastakentoconfirmedtheproductionofhydroxylradicalsintheFeⅢ-fumaratesystemunderUVradiationanditwashelpfulforthestudyofOrangeⅡdecolorizationmechanism.KeywordsFeⅢ-fumarateOrangeⅡphoto-oxidation·OHkinetics1-45-6.7-8.Fe-OH9-10、Fe-11-15、Fe-16-17.Fe-.FeⅢ-182h90%FeⅢ-CrⅥ19CrⅥ100%.Fe-·OH·OHFe-1UVFeⅢ-Ⅱ·OH720-21.、22.Ⅱc〔FeⅢ〕、ρⅡcpHⅡFeⅢ-·OHFeⅢ-ⅡFeⅢ-.11.1FeCl3·6H2O、ⅡOrangeⅡ、fumarate、coumarinAR..UV1800pHs-3CMulti340iWTWGermanyFC204500W79-1L5SSPerkinElmerMultiNC3100.1.2c〔FeⅢ〕1mmolLc10mmolLρⅡ1gLHClNaDHpH.500WUV5min50min.Ⅱ484nm23FeⅢ-Ⅱ·OH.ρⅡρⅡ1ⅡE%E=1-CtC0×1001C0ρⅡCttminρⅡmgL.22.1Ⅱc〔FeⅢ〕25μmolL、c250μmolL、ρⅡ25mgLpH3Ⅱ1.1—Ⅱ+UV2—FeⅢ++Ⅱ+UV3—FeⅢ++Ⅱ4—+Ⅱ+UV5—FeⅢ+Ⅱ+UV.1ⅡFig.1ContrastexperimentsofthedecolorizationofOrangeⅡ1Ⅱ0.FeⅢ50minⅡ93%.FeⅢⅡ①FeⅢFe-OH·OH1024②5③FeⅢ·OHⅡ.Ⅱ150min76%.ⅡFeⅢ-Ⅱ.FeⅢ、FeⅢ-2.FeⅢ-FeⅢⅡ.2.2pHc〔FeⅢ〕25μmolL、c250μmolL、ρⅡ25mgL38281—2—FeⅢ3—FeⅢ-.2Fig.2UltravioletabsorptionspectraofFeⅢfumarateandFeⅢ-fumaratecomplexespH3、4、5、73.pH1—72—53—44—3.3pHⅡFig.3EffectofpHonthedecolorizationofOrangeⅡ3pH3~7pHⅡ.pH3Ⅱ50min100%.pHFeⅢⅡⅡ·OH.2.3c〔FeⅢ〕250μmolL25mgLⅡFeⅢc〔FeⅢ〕10、25、4050μmolL.4c〔FeⅢ〕Ⅱ.c〔FeⅢ〕FeⅢ5.cc〔FeⅢ〕FeⅢ·OH、H2O2.FeⅢFe-OH·OH1024Ⅱ.c〔FeⅢ〕μmolL1—502—403—304—20.4c〔FeⅢ〕ⅡFig.4EffectofFeⅢinitialconcentrationsondecolorizationofOrangeⅡc〔FeⅢ〕μmolL1—502—403—254—10.5Fig.5UltravioletabsorptionspectraofFeⅢ-fumaratesystemindifferentconcentrations2.4ρⅡcρⅡcⅡ6、7.6ρⅡ25mgLc〔FeⅢ〕25μmolLcⅡc·OH.c250μmolL30minⅡ94%.7c〔FeⅢ〕25μmolL、c250μmolLⅡρⅡ.481UVFeⅢ-Ⅱc〔FeⅢ〕25μmolL、c250μmolL、ρⅡ10mgL、pH320minⅡ97%.cμmolL1—7502—5003—2504—100.6cⅡFig.6EffectoffumarateinitialconcentrationsondecolorizationofOrangeⅡρⅡmgL1—402—303—204—10.7ρⅡⅡFig.7EffectofOrangeⅡinitialconcentrationsondecolorizationofOrangeⅡ2.5〔lnC0C=kt〕ρⅡt0~50min1.1c〔FeⅢ〕、cρⅡⅡc〔FeⅢ〕、c0.08399、0.08056min-1ρⅡ.c〔FeⅢ〕25μmolL、c250μmolL、ρⅡ10mgL0.11296min-1.1ⅡTable1KineticsfittingprocessforthedecolorizationofOrangeⅡkmin-110lnC0C=0.07156t0.07156c〔FeⅢ〕μmolL25lnC0C=0.07356t0.0735640lnC0C=0.07646t0.0764650lnC0C=0.08399t0.08399100lnC0C=0.07489t0.07489cμmolL250lnC0C=0.07941t0.07941500lnC0C=0.08650t0.08650750lnC0C=0.08056t0.0805610lnC0C=0.11296t0.11296ρⅡmgL20lnC0C=0.08942t0.0894230lnC0C=0.07709t0.0770940lnC0C=0.05058t0.050582.6·OH·OH7-·OH2325-26.c〔FeⅢ〕25μmolL、c250μmolL5mL5×10-3molLpH3.7-Ⅱ8、9.min1—02—23—44—65—86—107—12.87-Fig.8Fluorescencespectrumof7-hydroxylcoumarininthephotooxidationreaction87-FeⅢ-·OH0~12minⅡ.7-COH2O5828min1—02—53—104—155—206—307—50.9ⅡFig.9UltravioletabsorptionspectraofOrangeⅡsolutionasafunctionofirradiationtime7-27-29.Ⅱ、—NN—3.UV-Vis484nm3310nm230nm30.9484nmFeⅢ-Ⅱ.310230nm.FeⅢ-Ⅱ60minTOC59.91%60.0%.FeⅢ-ⅡCO2H2O.3aFeⅢ-Ⅱc〔FeⅢ〕cρⅡ.bpH3~7ⅡpH3.c〔FeⅢ〕25μmolL、c250μmolL、ρⅡ25mgL、pH350minⅡ100%60minTOC60%.cⅡ.c〔FeⅢ〕cρⅡ.dFeⅢ-·OHⅡ.References1HANKeliHEGuozhong.PhotochemistryofarylhalidesphotodissociationdynamicsJ.JournalofPhotochemistryandPhotobiologyCPhotochemistryReviews20078255-66.2MELLOUKIAMUYujing.OntheatmosphericdegradationofpyruvicacidinthegasphaseJ.JournalofPhotochemistryandPhotobiologyAChemistry200315723295-300.3ABRAHAMHRICHARDMGEERTKetal.TheUV-VISabsorptioncrosssectionsoftheα-dicarbonylcompoundspyruvicacidbiacetylandglyoxalJ.JournalofPhotochemistryandPhotobiologyAChemistry200114612319-27.4ZUOYuegang.KineticsofphotochemicalchemicalcyclingofironcoupledwithorganicsubstancesincloudandfogdropletsJ.GeochimicaetCosmochimicaActa199559153123-3130.5.UVX-3BJ.200618180-84.FENGXianghuaFUTingboDINGShiminetal.DiscolourationofreactiveredX-3BsolutioninducedbypyruvateUVsystemJ.ChemicalResearchandApplication200618180-84.6HUANGSWCHIANGPNLIUJCetal.Chromatereductiononhumicacidderivedfromapeatsoil-explorationoftheacti